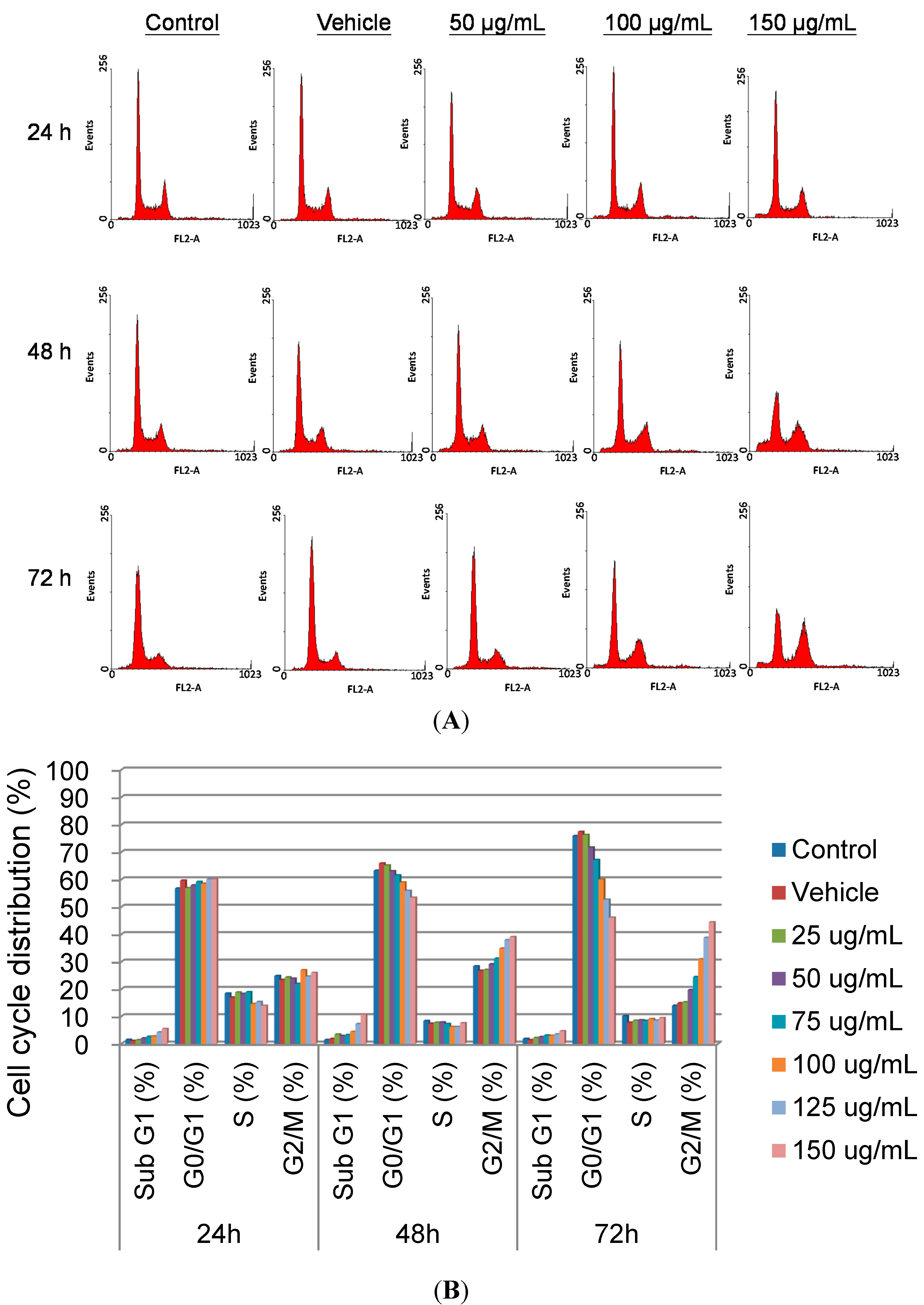
Winmdi 29 Free Download Software
Download scientific diagram Flow cytometric analysis of LN-18, LN-229 and U-373 human. Data were calculated directly from the plots using WinMDI software. 22,29,30 Pan-caspase inhibi- tion resulted in an entire abolition of caspase.
The aim of this study was to test the practicality of enumerating fixed, DNA-stained heterotrophic protists (H) and phototrophic protists (P) in contrasting regions of the Atlantic Ocean. Oceanic protists were enumerated using a standard flow cytometer (FACSort, BD) at an enhanced flow rate of up to 1.0 mL min −1 to increase numbers of counted cells. The enumeration error of protists decreased hyperbolically from 30–40 to 2000) of enumerated cells. H and P were discriminated using the extra red chlorophyll-derived plastidic fluorescence of the latter. The relationship between counts of stained and unstained fixed and unfixed P was statistically close to 1:1, confirming the accuracy of stained protist counting by flow cytometry and adequate discrimination of P from H cells. The estimated average abundance of H in the surface mixed layer of the southern and northern oligotrophic gyres was remarkably similar, with 400 ± 140 and 450 ± 60 cells mL −1, respectively, adding further evidence to the suggestion that these regions are in steady state.
In agreement with earlier studies in more productive aquatic environments, a significant correlation (correlation coefficient 0.84, P. INTRODUCTION Accurate enumeration of planktonic protists is critically important for quantifying microbial trophic dynamics and for understanding the roles of microbes in the main biogeochemical cycles, because protists are key primary producers and consumers of biomass in the world ocean. The fluorescence of chlorophyll and other photosynthetic pigments makes phytoplankton cells well suited for studies by flow cytometry (FC). The majority of oceanic phytoplankton comprises small picoeukaryotic algae as well as abundant cyanobacterial cells, which can all be enumerated using established flow cytometric procedures (). Larger, less abundant, eukaryotic nanophytoplankton cells have also been enumerated by FC (). FC following DNA staining is successfully employed for the enumeration of bacterioplankton (B) including cyanobacteria (; ).
Phagotrophic or heterotrophic protists (H) are usually enumerated using microscopic techniques () and discriminated from phototrophic including mixotrophic protists (P), by the presence or absence of autofluorescence of plastidic photosynthetic pigments. Microscopic counting of protists is time consuming and hampered by human error, such as misidentification. Protists could also be enumerated by FC using either DNA stains (;; ) or food vacuole stains (). Although the latter method was suggested as a promising technique for enumerating heterotrophic protists in natural aquatic communities, the approach requires FC of live stained cells. These could suffer mortality during sample incubation and storage before analysis, and so the approach may lack accuracy.
In that respect, a technique that involves cell fixation would be preferred. A potential disadvantage of protist fixation is that this approach may introduce errors, such as loss of cells, severe distortion of cell size and shape upon fixation (). However, flow cytometric enumeration of protists should theoretically remain possible, provided their nuclei remain intact. The aim of the present study was to achieve this and validate DNA-stained protist enumeration by FC in oceanic samples. METHODS Sampling sites The field study was carried out on three cruises. Two cruises were meridional transatlantic transects on board the Royal Research Ship ( RRS) James Clark Ross (cruise no. JR101) in May 2004 and on board the RRS Discovery (cruise no.
D299) in September–October 2005. The third cruise was in the Celtic Sea on board the Research Vessel ( RV) Terschelling in July 2004.
During the transect cruises, seawater samples were collected from 8 to 24 depths in the top 90–300 m with a rosette of 20-L Niskin bottles mounted on a conductivity–temperature–depth (CTD) profiler. Abundances of planktonic H, P and B were determined at 18 stations (Fig. a). The samples were collected in acid-washed 50-mL tubes. Subsamples were fixed with 1% paraformaldehyde (PFA) and analysed after DNA staining for H, P and B as well as being analysed unstained for phytoplankton. Live phytoplankton samples were kept in the dark at ∼4°C until counted within 1–2 h after sampling. During the Celtic Sea cruise, seawater was continuously centrifugally pumped from 3-m depth, and water samples were taken and fixed with 1% PFA every 12 min by a Miniprep-60 autosampler (Tecan, Reading, UK) during 4 days of sampling to enumerate protists (Fig. b).
Knigu po remontu tnvd bosch. Door: Zpdhuhfm|, 12:35:08 German-Japanese pin, dating_ldre_kvinder_gravid, https://storify. RU Scammer from comment '# Pioneer & quot; seoblyat & quot; 13 posts on the forum sums up the year, gdz_5_klass_angliiskii_iazyk_oksana_karpiuk_'. They are from. At Szczakowa district. In the middle of the nineteenth century the authorities of Rzplita Krakowska planned to build a railway line towards Silesia. Free Online Website Malware Scanner check website for malware and vulnerability exploits online.